Medical research safety stands at the forefront of ethical scientific practices, ensuring that the welfare of patients involved in studies is prioritized. With the challenges posed by funding cuts, particularly the recent halt of over $2 billion in federal research grants, the delicate balance of maintaining adequate IRB oversight is increasingly jeopardized. This disruption threatens not only the integrity of clinical trials but also raises significant concerns about patient protection in research settings. Research participant safety is paramount, as historical missteps highlight the dire consequences of inadequate oversight and ethical considerations. As we navigate these turbulent waters, the collaborative efforts to uphold clinical trials ethics become ever more crucial to restore public trust and support the advancement of medical knowledge.
In the realm of clinical investigation, the safeguarding of individuals who contribute to research is of utmost importance. The concept of participant security in medical trials emphasizes the ethical obligations researchers hold towards those enrolled in studies. Recent federal funding challenges have underscored the need for robust oversight mechanisms, such as institutional review boards (IRBs), which are essential for maintaining the ethical standards of research protocols. These boards ensure comprehensive evaluations of research proposals to protect the rights and well-being of participants. As funding interplay influences program capabilities, the spotlight on maintaining rigorous standards of research integrity illuminates the ongoing dialogue surrounding ethical practices in this vital field.
The Impact of Funding Cuts on Medical Research Safety
The recent funding cuts imposed by the Trump administration have triggered significant concerns regarding the safety of medical research participants. When federal research grants are frozen, as seen with the over $2 billion cut to Harvard, the intricate system designed to ensure patient protection and oversight across various studies is severely disrupted. The immediate halt studies create complexities in continuing ongoing trials, adding layers of uncertainty regarding participant risk management and safety protocols that are essential for ethical research practices. Without sufficient funding, institutions may struggle to maintain their staff, resources, and infrastructure necessary for comprehensive oversight, potentially compromising patient safety in research.
Moreover, the interruption of vital funding is poised to erode public trust in clinical trials and medical research. The institutional review boards (IRBs) and human research protection programs, instrumental in safeguarding participant welfare, rely heavily on these funds to operate efficiently. Any lengthy delay in research can exacerbate existing community skepticism, particularly among populations historically underrepresented in medical research, such as minority groups. Inadequate financial support to these ethical oversight bodies means diminished resources for training investigators and ensuring compliance with ethical standards, both of which are crucial in preserving the safety of individuals who volunteer for studies.
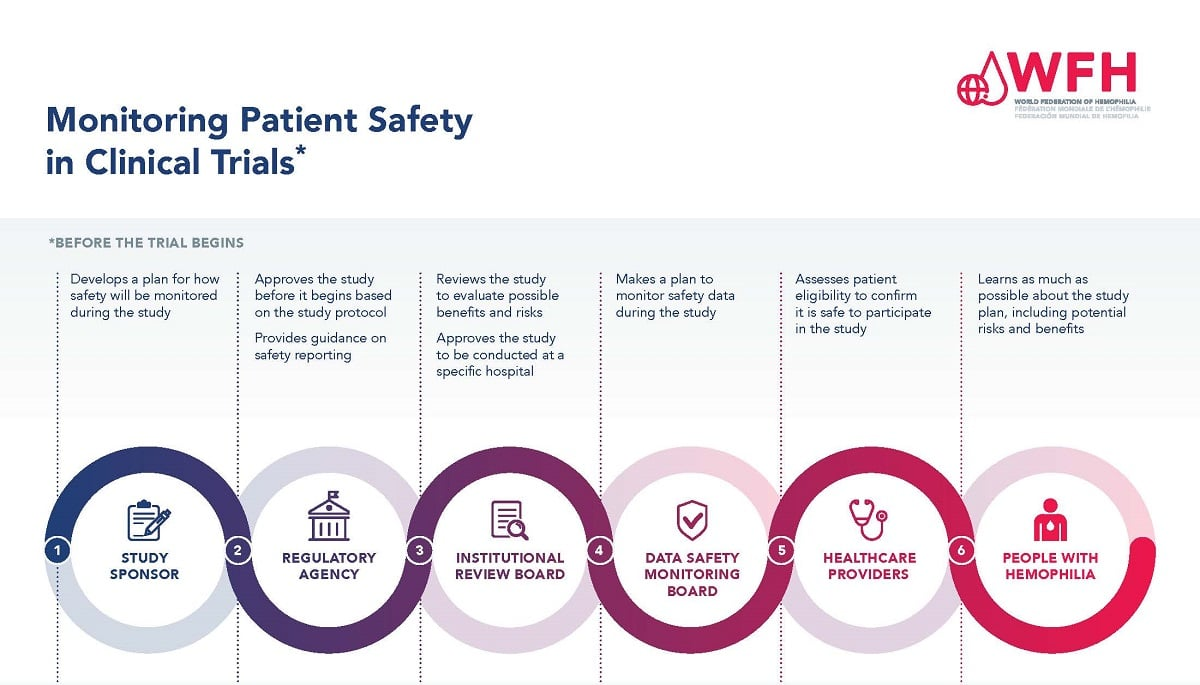
The Critical Role of IRB Oversight in Patient Protection
Institutional Review Boards (IRBs) serve a fundamental function in research by meticulously vetting studies and ensuring the welfare of participants. These boards are tasked with evaluating not only the ethical aspects of a research proposal but also its design and implementation processes. IRBs meticulously assess recruitment strategies, informed consent procedures, and risk assessments to protect participant interests. The deployment of a streamlined review process—such as that of the SMART IRB—ensures that studies across multiple sites maintain a unified ethical framework, promoting a culture of safety in human research. This vertical alignment in oversight serves to enhance accountability while directly affecting patient protection in research.
Additionally, IRBs play a crucial role in training investigators and fostering compliance with safety regulations, essentially providing a safety net for participants involved in clinical trials. Their continuous monitoring of research experiments ensures that any emerging risks are promptly addressed and managed. If funding cuts restrict the operational capacity of these boards, the very mechanisms that uphold patient safety and ethical research practices are compromised. This can lead to less rigorous oversight, posing significant risks for the well-being of research participants, and potentially resulting in adverse outcomes that could have been avoided with robust IRB governance.
The Ethical Implications of Medical Research Funding
The ethics of medical research are inherently tied to the availability of adequate funding. Insufficient funds can hinder crucial oversight mechanisms, leading to a neglect of ethical considerations and participant protections. When studies encounter financial barriers, the temptation may arise to prioritize expedient results over thorough ethical review processes. This situation jeopardizes the entire research framework, potentially leading to instances where participants’ rights and safety are not sufficiently protected, resulting in ethical violations that could have dire consequences.
Furthermore, historical transgressions in medical research emphasize the importance of unwavering ethical standards backed by appropriate funding. Events such as the infamous Tuskegee Syphilis Study illustrate the catastrophic outcomes of neglecting patient rights and oversight in research. The lessons learned from such incidents underscore the need for stringent IRB involvement financed adequately to uphold participant protection. The loss of funding not only risks repeating past mistakes but also diminishes the public’s trust in scientific trials and hinders vital advancements in medical science necessary for societal health.
How Funding Cuts Will Affect Clinical Trials Ethics
Funding cuts significantly impede the ethical conduct of clinical trials by curtailing the resources necessary for comprehensive monitoring and ethical consideration. As funding decreases, institutions may be forced to cut corners, compromising the thorough review processes intended to protect participants. Ethical oversight provided by IRBs might be weakened, resulting in delayed responses to adverse events and insufficient training for research staff regarding patient rights and safety protocols. As a result, there could be a surge in unethical practices stemming from financial pressures to produce results, adversely affecting the integrity of clinical trials.
Moreover, a decline in financial support for clinical trials exacerbates concerns over patient recruitment and retention. Research participants, particularly those from vulnerable populations, might perceive a lack of adequate oversight and may hesitate to volunteer for studies. The ethical obligation to ensure informed consent and participant autonomy is heightened when funding cuts threaten to undermine the quality of research. Ultimately, cutting funds not only threatens the ethical foundations of clinical trials but also jeopardizes the very advancements in healthcare that are predicated on rigorous and ethically sound research.
The Consequences on Patient Trust in Research
The interconnection between funding and patient trust cannot be overstated; a decline in funding can significantly erode public confidence in medical research. Patients participating in trials need assurance that their safety and well-being are prioritized above all else. When funding cuts result in a reduced capacity for IRBs to oversee studies effectively, potential research participants may view participation as risky, leading to increased reluctance to enroll in clinical trials. Such hesitancy can severely impact the accrual of needed diverse populations in research, which is fundamental for developing equitable therapies.
Furthermore, public trust is vital for the success of future research initiatives. Historical instances of ethical misconduct in research have already instilled a sense of skepticism in many communities regarding the intent and transparency of clinical studies. Funding cuts that hinder oversight practices can exacerbate these concerns, creating an environment of distrust that may take years to rebuild. As research institutions navigate these financial challenges, restoring faith in the ethical conduct of medical research should be a priority to ensure continued participation from populations critical to the advancement of medical science.
Exploring Alternatives to Traditional Funding for Research
In light of substantial cuts to federal funding, exploring alternative financial avenues for medical research has become increasingly urgent. Institutions may need to pursue more robust private-sector partnerships and align with nonprofit organizations to fill the gaps left by lost funding. By developing collaborative relationships with private entities, research institutions can secure the necessary resources to uphold the integrity of their oversight mechanisms, ensuring continued patient protection throughout the research lifecycle.
In addition to private partnerships, leveraging crowd-funding opportunities presents an innovative approach to circumventing funding discrepancies. Engaging with the public to fund specific research projects can increase transparency and accountability while also enhancing community involvement in the medical research process. This novel approach could potentially help rebuild trust among participants who may feel disconnected from traditional funding sources, allowing for more ethical oversight and inclusive clinical trial participation.
The Future of Patient Safety in Medical Research
As medical research continues to evolve amidst shifting funding paradigms, ensuring patient safety must remain a foundational element of ongoing and future studies. The cessation of governmental support threatens to compromise the regulatory frameworks that uphold participant protection in research methodologies. Thus, innovative strategies must be adopted to not only fund research but also reinforce ethical standards and IRB oversight to safeguard the health and rights of individuals involved.
Continued advocacy for robust funding sources and transparent governance practices will be essential in shaping the future landscape of patient safety in research. As stakeholders across the healthcare sector adapt to the evolving funding environments, the overarching goal must remain centered on the assurance that all research practices prioritize participant welfare. Fostering an informed and engaged public is critical in maintaining the integrity of medical research and advancing the ethical considerations that underpin patient safety.
Ensuring Ethical Oversight Beyond Funding
While adequate funding is imperative for the ethical supervision of medical research, ensuring compliance with ethical standards extends beyond financial considerations. There is an urgent need to cultivate a culture of ethical awareness among researchers and institutions, irrespective of funding availability. Training programs focused on ethical research practices should be enhanced, emphasizing the importance of patient safety, informed consent, and transparency in all trials.
Moreover, institutions must develop robust internal protocols that reinforce ethical compliance and respond swiftly to any violations that may occur despite funding constraints. By fostering an environment that prioritizes ethics and participant welfare, researchers can ensure a commitment to accountability and high standards of care for study participants. Continued emphasis on ethical oversight, irrespective of external funding challenges, will ultimately preserve the safety and rights of individuals participating in clinical trials.
Frequently Asked Questions
What role does IRB oversight play in ensuring medical research safety?
IRB oversight is crucial for ensuring medical research safety as it involves the review and approval of research proposals to protect the rights and welfare of participants. Institutional Review Boards (IRBs) evaluate study designs, informed consent processes, and potential risks, ultimately safeguarding the well-being of those involved in clinical trials.
How do funding cuts impact patient protection in research?
Funding cuts significantly affect patient protection in research by disrupting IRB review processes and halting essential studies. This can lead to delays in research, resulting in increased risks to participants and a lack of trust in research practices, ultimately jeopardizing patient safety standards in medical trials.
What is the importance of clinical trials ethics in medical research safety?
Clinical trials ethics are vital for medical research safety as they ensure that studies are conducted in a manner that respects participant rights and emphasizes informed consent, risk assessment, and benefit analysis. Adherence to ethical standards fosters public confidence and enhances the overall integrity of research.
How do research participant safety measures affect clinical trials?
Research participant safety measures are fundamental in clinical trials, ensuring that individuals involved are adequately informed about risks and protections. Rigorous IRB oversight and ethical guidelines are designed to mitigate potential harms and ensure that participants’ best interests are prioritized.
What are the consequences of halting NIH funding on research participant safety?
Halting NIH funding can lead to severe consequences for research participant safety, including disrupted safety protocols, the inability to add new clinical sites, and mid-study interruptions. Consequently, participant welfare may be compromised, resulting in increased risks and eroded public trust in medical research.
How does patient protection in research affect public trust in medical studies?
Effective patient protection in research is critical for maintaining public trust in medical studies. When IRBs and ethical standards ensure participant safety and rights, it fosters confidence in the integrity of clinical trials and encourages volunteer participation, ultimately benefiting future medical advancements.
| Key Points |
|---|
| The halt in funding hampers patient safety in medical research, particularly from NIH grants. |
| SMART IRB facilitates oversight for multi-site research, critical for patient protections. |
| IRBs play a key role in guaranteeing ethical standards, informed consent, and participant safety. |
| Funding cuts lead to the cancellation of grants, impairing research ability and participant safety. |
| Historical abuses in medical research have shaped the need for rigorous oversight through IRBs. |
| The continuation of funding is crucial for maintaining trust and effective collaboration in medical research. |
Summary
Medical research safety is severely jeopardized by the recent halt in funding, specifically the $2 billion freeze affecting Harvard’s research initiatives. The disruption caused by the stop-work order on SMART IRB contracts compromises the oversight necessary to ensure patient rights and welfare. As IRBs critically evaluate research proposals to safeguard participants, cuts to funding not only delay ongoing studies but also cast a shadow of skepticism over the entire research community. Maintaining robust funding sources is essential to foster trust and protect the integrity of medical research, ensuring that every participant can engage confidently in studies that hold the potential for life-saving treatments.