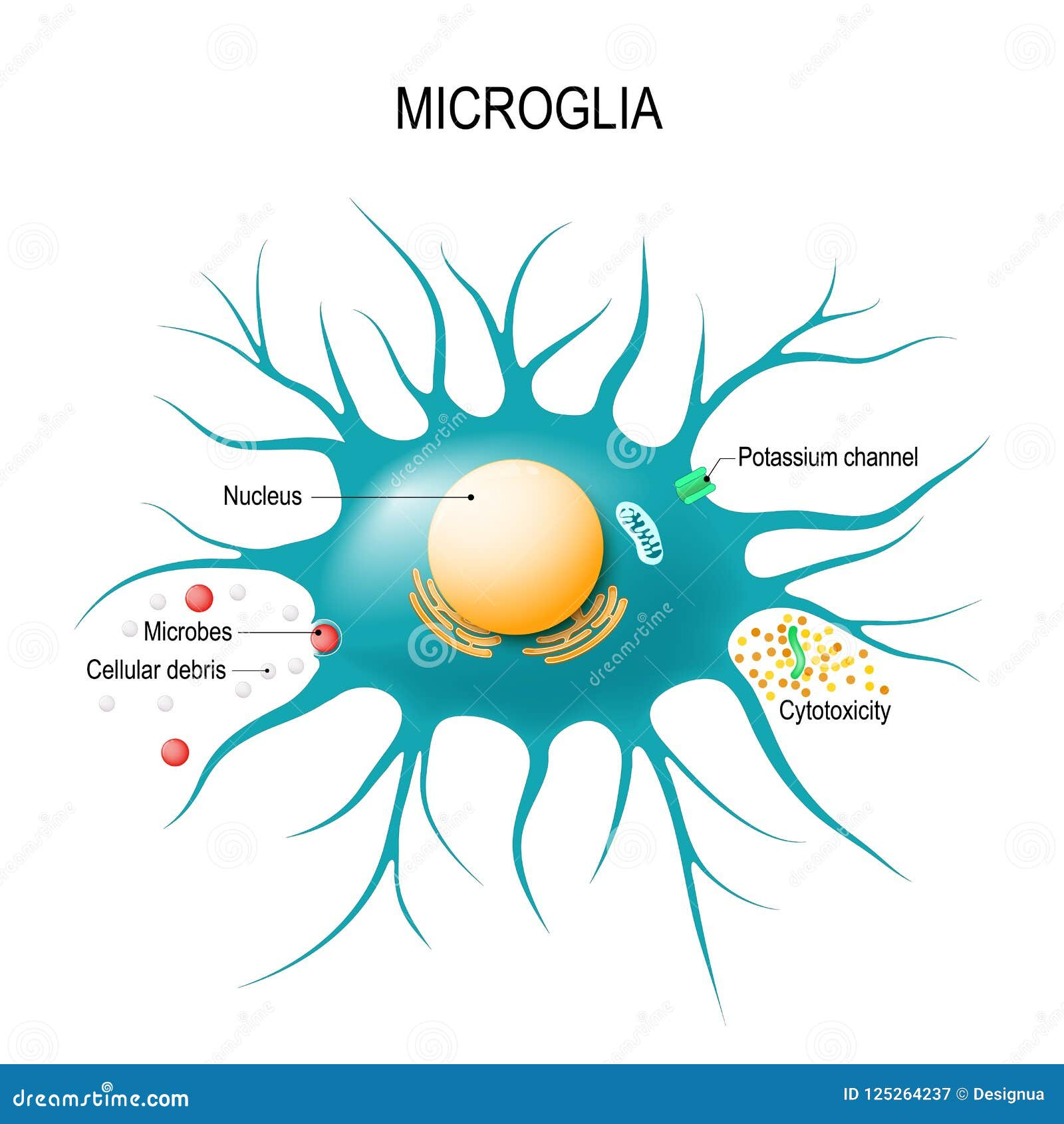
Microglial cells, the brain’s resident immune defenders, play a pivotal role in maintaining neural health and homeostasis. These specialized cells are not only responsible for monitoring and responding to brain health challenges, but they also engage in synaptic pruning, a process crucial for effective neuronal communication. Leading research efforts by neuroscientist Beth Stevens have shed light on how these microglia can sometimes malfunction, leading to detrimental effects and worsening conditions like Alzheimer’s disease and other neurodegenerative diseases. Understanding the behavior of microglial cells is essential for developing innovative treatments and biomarkers in Alzheimer’s research, highlighting the intricate relationship between brain health and immune function. With the prevalence of neurodegenerative diseases on the rise, uncovering the mysteries surrounding microglial activity has never been more urgent or relevant.
In the realm of brain health, microglial cells represent a critical aspect of the brain’s immune system, acting as vigilant protectors against injury and disease. These pivotal cells not only carry out the necessary task of synapse elimination—often referred to as synaptic pruning—but also play a significant role in the broader context of neurobiology and cognitive function. Recent advancements in understanding these immune cells have been largely credited to innovative research from scientific leaders like Beth Stevens, particularly in relation to Alzheimer’s and other cognitive disorders. The exploration of these brain-resident immune cells informs the ongoing fight against neurodegenerative diseases, providing valuable insights into potential therapeutic avenues. As we continue to uncover the complexities of the immune landscape within our brains, the promise of enhanced treatment options becomes increasingly tangible.
Understanding Microglial Cells in Alzheimer’s Research
Microglial cells play a crucial role in maintaining the health of the brain by acting as its primary immune defense. In recent years, research has highlighted their function in synaptic pruning, the process by which unnecessary or damaged synapses are removed. Beth Stevens, a prominent neuroscientist, emphasizes that while microglia support normal neurodevelopment, their dysregulation can lead to significant issues in neurodegenerative diseases such as Alzheimer’s. This critical insight underscores the importance of continuing investigations into how these cells operate within the broader context of the brain’s immune system.
Moreover, Stevens’ research at the Stevens Lab has revealed that aberrant microglial activity can contribute to the onset and progression of various neurodegenerative diseases, highlighting their dual role. While they are essential for clearing damaged cells and supporting neural health, their overactivation can exacerbate conditions like Alzheimer’s by promoting synaptic loss instead of repair. These findings open new avenues for potential therapeutic interventions targeting microglial pathways, ultimately aiming to slow or halt the progression of diseases that currently lack effective treatments.
The Connection Between Synaptic Pruning and Neurodegenerative Diseases
Synaptic pruning, a natural and necessary process for cognitive development, has gained attention for its implications in neurodegenerative diseases. As researchers like Beth Stevens continue to explore this phenomenon, the understanding of how improper synaptic pruning may lead to conditions such as Alzheimer’s is becoming clearer. Abnormal pruning activity indicates that microglia may selectively remove synapses that are not necessarily faulty, which contributes to cognitive decline and memory loss in affected individuals. This research signals a shift in how the scientific community perceives the brain’s immune responses during the onset of neurodegeneration.
Furthermore, understanding this connection allows for the establishment of biomarkers that could predict the early stages of Alzheimer’s and other cognitive disorders. By identifying specific markers that indicate aberrant microglial activity, scientists can develop interventions that could prevent or slow the degenerative processes involved in diseases like Alzheimer’s. Ongoing research into synaptic pruning and its regulation by microglial cells emphasizes the necessity of integrating findings from basic science to clinical applications, promising a future where treatment modalities can shift from reactive to preventative.
The Role of the Brain’s Immune System in Fighting Neurodegenerative Disease
The brain’s immune system, primarily orchestrated by microglial cells, plays a pivotal role in addressing neurodegenerative diseases. Neuroinflammation, often observed in conditions like Alzheimer’s, can be modulated by understanding and manipulating the actions of microglia. Beth Stevens’ work expands the narrative around neuroinflammation, showcasing that rather than being merely a destructive force, microglial activation can also initiate protective mechanisms if properly regulated. This perspective shift is essential in developing new strategies for treating diseases characterized by both inflammation and neurodegeneration.
Researchers are continually investigating how to harness the brain’s immune capabilities to alleviate the burden of neurodegenerative diseases. By focusing on the balance between activation and suppression of microglial responses, innovative therapeutic approaches can be designed. This includes not only pharmacological intervention but also potential lifestyle changes that could promote healthy brain functions, such as diet and exercise, which have shown promise in modulating neuroinflammatory responses. Understanding how to effectively engage the brain’s immune system provides hope for improved outcomes for millions affected by these debilitating diseases.
Innovations Driven by Basic Science in Neuroscience
Beth Stevens emphasizes the significance of basic science as the foundation for transformative research in neuroscience. Her journey through the complexities of microglial research reveals how fundamental questions can yield profound insights into neurodegenerative diseases. This work is pivotal because breakthroughs often stem from curiosity-driven studies, which explore unknown territories. For instance, examining the role of microglial cells in the visual systems of mice provided a model to understand their function far beyond initial expectations, linking basic research to clinical outcomes.
The importance of funding from organizations like the National Institutes of Health cannot be overstated in this context. Such support allows researchers to pursue their scientific inquiries without the immediate pressure of commercial viability. As Stevens points out, many may view investigations that seem distant from clinical applications as unimportant; however, these foundational studies are crucial for paving the way for future therapies. By continuing to support basic science in fields like Alzheimer’s research, the potential for significant advances in treatments and understanding of diseases remains promising.
Perspectives from Alzheimer’s Research: A Personal Journey
The personal journey of researchers like Beth Stevens inspires a greater understanding of the scientific process in Alzheimer’s research. As she navigated her early career, she followed leads that many may have considered irrelevant, yet her pursuit of knowledge led to groundbreaking discoveries about microglial cells and their function in synaptic pruning. This highlights how passion and curiosity are powerful drivers in the realm of science, often leading to unexpected breakthroughs that challenge long-held assumptions.
Stevens recounts the initial skepticism surrounding her research on microglia, demonstrating the importance of resilience and vision in scientific inquiry. Each experiment, regardless of its immediate applicability, contributed to a collective body of knowledge that could one day unravel the complexities of neurodegenerative diseases. Her work serves as a reminder that progress often emerges from the dedication to understanding the intricacies of the brain, reinforcing the belief that every discovery, no matter how small, is a step toward combating issues such as Alzheimer’s.
New Biomarkers and Medicines in Neurodegenerative Disease Treatment
Amid the challenges posed by Alzheimer’s and other neurodegenerative diseases, researchers are uncovering promising new biomarkers that could revolutionize diagnosis and treatment. Beth Stevens’ research into microglial activity has shed light on potential indicators that signify disease progression. Identifying these biomarkers is essential for developing targeted therapies aimed at mitigating the effects of Alzheimer’s, enabling healthcare providers to tailor treatment plans for individuals based on their unique neurobiological markers rather than a one-size-fits-all approach.
Furthermore, these new biomarkers hold the potential to unlock innovative medicinal treatments focused on recalibrating microglial functions. By understanding how to modulate microglial activity, scientists may be able to slow down or reverse the harmful synaptic pruning processes linked to neurodegeneration. The continuous evolution of research in this area is crucial, as it may lead to breakthroughs that improve patient outcomes and quality of life for millions affected by Alzheimer’s and other neurodegenerative disorders.
Taking Insights from Animal Models to Human Therapy
Research using animal models has been instrumental in advancing our understanding of human neurological disorders. The investigation of microglial cells in mice, as performed by Stevens and her team, has provided critical insights into their role within the brain’s immune system. Such studies allow researchers to explore complex mechanisms of disease in a controlled environment, enabling them to unearth discoveries that can eventually inform human clinical trials and therapies. The transition from animal models to human applicability underscores the significance of foundational research.
Understanding the specific actions of microglia in health and disease can enhance the translation of findings from animal studies into effective treatments for human patients. For instance, insights into synaptic pruning dynamics in rodent brains could guide therapeutic interventions aimed at modulating microglial activity in Alzheimer’s patients. As the scientific community continues to leverage animal models, the goal remains to unlock critical pathways that could lead to innovative treatment methodologies tailored to counteract the detrimental effects of neurodegenerative diseases.
Funding and Support for Alzheimer’s Innovations
The ongoing pursuit of new knowledge in Alzheimer’s research heavily relies on sustained funding and support from institutions like the NIH. Beth Stevens’ experience illustrates the role of federal funding in enabling scientists to develop cutting-edge research without the constraints of immediate commercialization. This foundational financial support allows for exploratory studies that may not seem immediately relevant but are necessary for uncovering the complexities of the brain and its immune responses, particularly concerning diseases like Alzheimer’s.
Stevens advocates for ongoing investment in basic science research, emphasizing that breakthroughs in understanding the brain’s immune system are foundational for future innovations in disease treatment. As the landscape of neurological research evolves, adequate funding is essential to empower scientists to tackle some of the most pressing health crises faced by society today. Supporting research initiatives not only fosters creativity and exploration but ultimately contributes to the development of new therapies and strategies in the fight against Alzheimer’s.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial to Alzheimer’s research as they act as the brain’s immune system, monitoring and responding to signs of damage or disease. In Alzheimer’s, studies reveal that these cells contribute to neuroinflammation and may engage in aberrant synaptic pruning, which can lead to neuron degradation. Understanding their function is essential for developing potential therapies.
How do microglial cells influence neurodegenerative diseases beyond Alzheimer’s?
Microglial cells influence several neurodegenerative diseases, including Huntington’s disease and Parkinson’s disease. Their role in synaptic pruning can become dysregulated, leading to excessive pruning of healthy synapses, which is detrimental to neural communication and health. This underscores the importance of targeting microglial function in neurodegenerative disease treatments.
What is the significance of synaptic pruning by microglial cells in brain health?
Synaptic pruning by microglial cells is vital for brain health as it helps eliminate unnecessary or damaged neuronal connections, thus enhancing neural network efficiency. Proper regulation of this process is essential to prevent conditions such as Alzheimer’s disease, where improper pruning can exacerbate cognitive decline.
Who is Beth Stevens and what is her contribution to understanding microglial cells?
Beth Stevens is a prominent neuroscientist known for her groundbreaking work on microglial cells and their role in the brain’s immune system. Her research at Boston Children’s Hospital and the Broad Institute has advanced our understanding of how microglia contribute to neurodegenerative diseases and has paved the way for potential biomarkers and treatments.
Can understanding microglial cells lead to new therapies for Alzheimer’s disease?
Yes, understanding microglial cells has the potential to lead to new therapies for Alzheimer’s disease. By uncovering the mechanisms behind their involvement in synaptic pruning and inflammation, researchers can develop targeted treatments to modify microglial activity, potentially halting or reversing disease progression.
What discoveries have been made regarding microglial cells and synaptic function?
Recent discoveries indicate that microglial cells play a critical role in synaptic function by selectively pruning synapses during development and in response to injury. However, disruptions in this process can lead to neurodegenerative diseases, highlighting microglial cells as key players in maintaining synaptic integrity.
How is microglial research shaping our understanding of the brain’s immune system?
Microglial research is revolutionizing our understanding of the brain’s immune system by revealing how these cells respond to neuroinflammation, clear debris, and regulate synaptic pruning. This knowledge is crucial for uncovering the pathogenesis of neurodegenerative diseases and developing effective treatments.
| Key Point | Description |
|---|---|
| Role of Microglial Cells | Microglial cells act as the brain’s immune system, essential for monitoring and responding to brain health. |
| Pruning Function | They assist in clearing dead or damaged cells and pruning synapses vital for neuronal communication. |
| Research Impact | Aberrant microglial pruning has been linked to Alzheimer’s and Huntington’s diseases, leading to potential biomarkers and treatments. |
| Stevens’ Contributions | Beth Stevens’ research has transformed the understanding of microglial function and its implications in neurodegenerative diseases. |
| Funding and Support | Vital funding from NIH and federal agencies has been essential to progress in microglial research. |
Summary
Microglial cells are crucial for brain health, serving as the brain’s immune defenders. Recent research by neuroscientist Beth Stevens highlights how these cells not only protect the brain but also play a significant role in neurodegenerative diseases like Alzheimer’s. Understanding their function, including their role in synaptic pruning, paves the way for new treatment approaches. Continued investigation into microglial cells will be essential for developing effective therapies and improving outcomes for millions affected by these debilitating conditions.