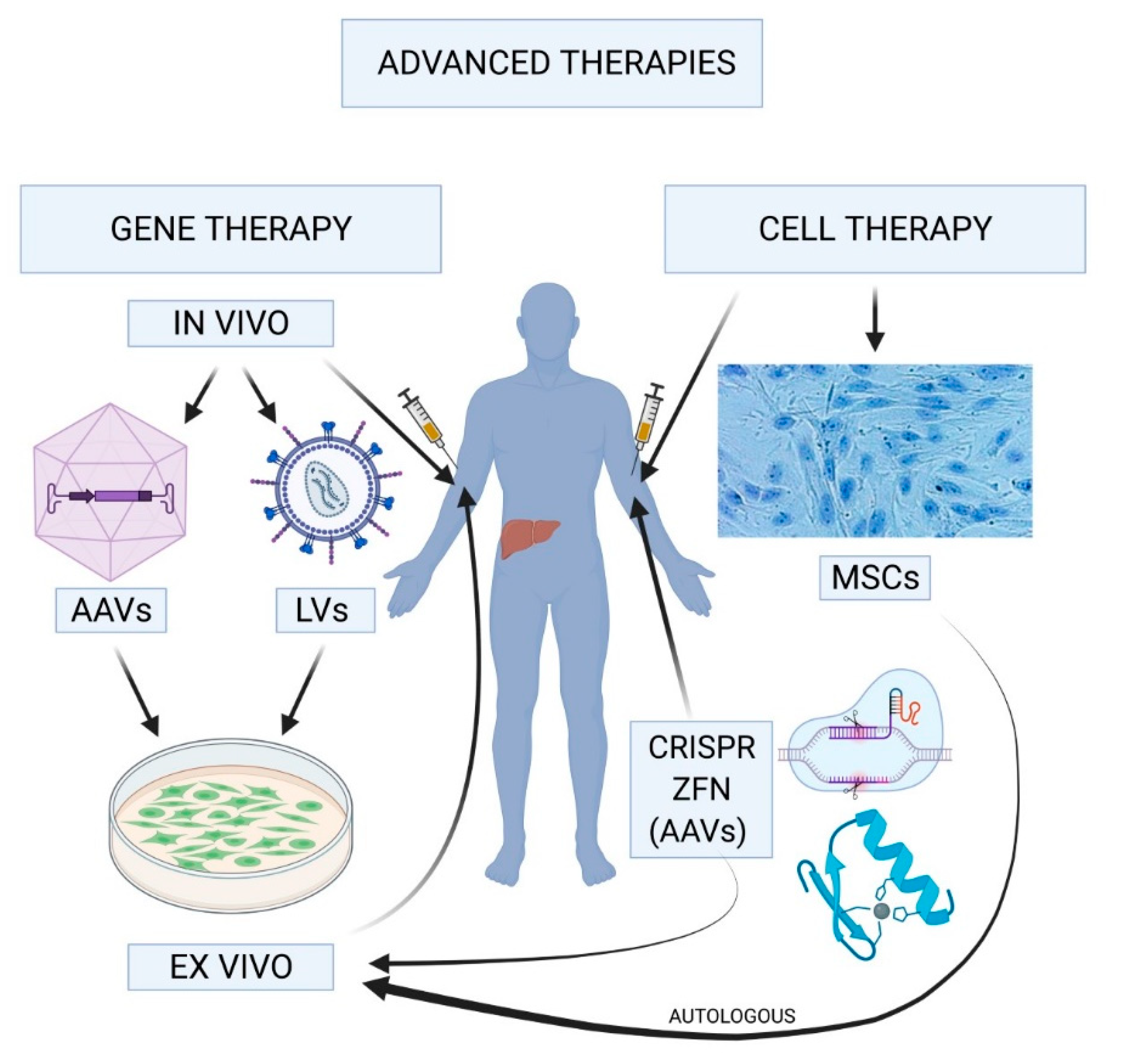
Gene therapy for hemophilia is revolutionizing the way patients manage this lifelong condition, offering a promising alternative to traditional hemophilia treatment methods. Specifically, Hemgenix gene therapy is making headlines as it provides a groundbreaking solution for individuals with hemophilia B, a form of this bleeding disorder. With its ability to deliver the necessary clotting factor directly to the liver, patients like Terence Blue are experiencing unprecedented relief from the constant need for injections and the accompanying worries. The benefits of gene therapy extend beyond just convenience; they hold the potential for a significant improvement in quality of life, allowing patients to engage more freely in daily activities. As advancements in medical technology progress, gene therapy may finally pave the way to a brighter future for those living with hemophilia.
The introduction of genetic modifications to address bleeding disorders marks a transformative era in hemophilia care, particularly through innovative approaches such as gene therapy. This method represents a shift from conventional treatments, including regular injections of clotting factors, to a more permanent solution aimed at correcting the underlying genetic defects. The Hemgenix therapy exemplifies this shift, aiming to enhance blood clotting in those affected by hemophilia B. As the medical field continues to explore the advantages of these advanced therapies, patients can hope for a future where they are less reliant on frequent treatments and can lead healthier, more active lives. Gene therapy not only signifies progress in hemophilia management but also ignites hope for a potentially life-changing cure.
Understanding Hemophilia B: A Lifelong Battle
Hemophilia B is a genetic disorder that primarily affects males, resulting from a deficiency in clotting factor IX. This condition can lead to spontaneous bleeding episodes and requires careful management to prevent severe complications. Patients like Terence Blue often face significant challenges, including frequent visits to the doctor, daily injections of clotting factors, and the constant fear of unmanageable bleeds. For many, the reality of living with hemophilia involves a lifetime of vigilance and medical intervention.
The psychological impact of hemophilia can be profound. Patients often feel isolated due to their condition, which can limit their ability to participate in physical activities or social events. Terence’s experience illustrates this struggle; despite achieving milestones in his life, he constantly had to navigate the social implications of his disorder. The advancements in treatment, like gene therapy, offer a glimmer of hope for a future where patients may not have to endure such significant restrictions due to their condition.
The Role of Gene Therapy in Hemophilia Treatment
Gene therapy represents a revolutionary advancement in the treatment landscape for hemophilia, particularly for those diagnosed with hemophilia B. The newly approved therapy, Hemgenix, uses a viral vector to deliver a healthy copy of the factor IX gene directly to liver cells, allowing the body to produce the missing clotting factor naturally. This innovative approach aims to reduce or eliminate the need for regular injections of clotting factors, which have traditionally been the cornerstone of hemophilia treatment.
The potential benefits of gene therapy are significant. For patients like Terence Blue, who recently received Hemgenix, the prospect of a more typical life without daily worry over bleeding episodes is life-changing. The treatment aims not only to improve the quality of life but also to enhance long-term health outcomes by allowing patients to maintain normal activities without the constant concern of bleeding complications. As more patients gain access to this therapy, the hope is to see a shift in how hemophilia is managed, moving towards a model that emphasizes a more independent lifestyle.
The Impact of Hemgenix on Patients
Hemgenix has emerged as a beacon of hope within the hemophilia community. Its relatively recent approval by the FDA marks a significant milestone in hemophilia treatment, showcasing the advancements in gene and cell therapies. Patients undergoing this treatment, including Terence Blue, have reported remarkable changes, such as increased factor IX levels and reduced dependency on traditional clotting factor therapy. These improvements not only alter physical health outcomes but also have profound implications for patients’ overall well-being and mental health.
However, the road ahead is not without challenges. With gene therapy priced at around $3.5 million, there are ongoing discussions regarding accessibility and insurance coverage. Patients and healthcare providers must advocate for pricing structures that enable broader access to these innovative treatments. As more therapies enter the market, the hope is that the cost will come down, allowing more individuals with hemophilia to experience the transformative benefits of gene therapies like Hemgenix.
Market Pressures Affecting Gene Therapy Adoption
Despite the promise gene therapies hold, market pressures present significant challenges. The high cost of therapies like Hemgenix creates barriers for many patients who could benefit from them. While some insurance policies might cover part of the cost, there’s no guarantee that all patients will receive the necessary financial support. This uncertainty can lead to hesitancy among potential patients considering cutting-edge treatments, which may limit their uptake even among those most in need.
Furthermore, the pharmaceutical market’s dynamics can influence the availability and viability of gene therapies. For instance, the recent withdrawal of Beqvez, another gene therapy for hemophilia B, demonstrates how fluctuating demand can impact treatment options. Companies must navigate the delicate balance between research investments and the commercial viability of their products, which can ultimately diminish the options available for patients with hemophilia. As the demand for effective treatments grows, it’s crucial for stakeholders to collaborate and ensure sustainable pathways for genetic therapies.
Revolutionizing Hemophilia Management Through Innovation
The introduction of gene therapy is revolutionizing how hemophilia is managed on a broader scale. With innovative treatments like Hemgenix set to change the trajectory of patient care, the paradigm is shifting from long-term, often cumbersome prophylactic strategies to potential one-time therapeutic interventions. Such advancements symbolically and literally represent a leap into the future of medicine, providing previously unimaginable solutions to patients.
With every successful treatment, cases like that of Terence Blue inspire hope and drive further research into new therapies. The excitement surrounding these groundbreaking treatments fosters a vibrant ecosystem of innovation, where scientists, doctors, and patients work in concert towards advancing care for hemophilia and other genetic disorders. The focus is now on harnessing the power of gene therapy to improve patients’ lives, hoping to turn treatment into a cure.
Life After Gene Therapy: What’s Next for Patients?
After receiving gene therapy, patients like Terence Blue often enter a new chapter in their lives. They experience a mix of hope and uncertainty, as the long-term effects of treatment continue to develop. For many, the immediate results are promising, with increases in factor IX levels and a reduction in spontaneous bleeding episodes. However, the real test lies in the coming months and years as they monitor their health and adapt to a new reality.
The implication of success extends beyond just the individual patient. As more people receive therapies like Hemgenix, the accumulated data on safety and efficacy will inform future research and treatment guidelines. The long-term follow-up of patients will be essential in understanding the full potential of gene therapy in transforming the landscape of hemophilia management. Discussions around supportive care and ongoing monitoring will play crucial roles in ensuring the sustainability of these groundbreaking advances.
Social Implications of Living with Hemophilia
Living with hemophilia has significant social implications for patients. As Terence Blue’s experiences illustrate, the need to explain one’s condition can create barriers in friendships and social interactions. Patients may experience isolation or exclusion from activities, especially when injuries are a concern. The stigma attached to visible conditions might discourage patients from participating in typical social sports or events, further enhancing feelings of loneliness.
However, the advent of therapies such as Hemgenix might change the narrative for many. By improving health outcomes, patients can engage more confidently in social settings, fostering deeper connections and participation in community activities. As gene therapy shifts the conversation from managing symptoms to potentially curing the underlying condition, it inspirationally promotes a more inclusive perspective on living with hemophilia.
The Future of Hemophilia Treatments: Ongoing Research and Innovation
The future of hemophilia treatments lies in the continuous innovation spurred by research and the integration of advanced technologies. With the approval of therapies like Hemgenix, researchers are exploring not only enhancements to existing gene therapy protocols but also investigating novel approaches that might apply to a broader range of genetic disorders. This ongoing commitment to advancing scientific understanding paves the way for new therapies that may further transform care.
Additionally, the increasing focus on personalized medicine offers promising avenues of research aimed at tailoring treatments to individual patient profiles. By harnessing the unique molecular characteristics of hemophilia in each patient, there is potential for even more effective and specialized therapeutic options. The resilience and dedication of the scientific community promise to unlock further innovations, ensuring the future of hemophilia management remains bright.
Empowering Patients Through Education and Advocacy
As gene therapies like Hemgenix become more prevalent, the importance of patient education and advocacy cannot be understated. Patients need accessible information about their condition, treatment options, and the potential risks and benefits associated with new therapies. Empowering patients to make informed decisions is critical for promoting adherence to treatment regimens and maximizing the potential benefits of innovative therapies.
Advocacy efforts also play a vital role in shaping the treatment landscape. Patient groups and healthcare organizations are instrumental in lobbying for increased research funding, insurance coverage, and access to cutting-edge therapies. By collectively raising awareness and promoting advocacy initiatives, the hemophilia community can ensure that patients receive the best possible care and support, ultimately improving health outcomes and quality of life for those living with this condition.
Frequently Asked Questions
What is gene therapy for hemophilia and how does it work?
Gene therapy for hemophilia, particularly hemophilia B, involves inserting a corrected copy of the defective gene responsible for producing clotting factor IX. This innovative treatment, such as Hemgenix, uses a virus as a vector to deliver the new gene directly into liver cells, where the body can begin producing the necessary clotting factor, potentially reducing or eliminating the need for regular clotting factor therapy.
What are the benefits of gene therapy for hemophilia compared to traditional treatments?
The primary benefits of gene therapy for hemophilia include the potential for long-lasting effects, possibly reducing the frequency of injections of clotting factor therapy from multiple times a week to infrequent or none at all. This could lead to an improved quality of life, as patients may experience fewer spontaneous bleeding episodes and enjoy greater freedom from daily medical routines.
How does Hemgenix gene therapy specifically help patients with hemophilia B?
Hemgenix gene therapy helps patients with hemophilia B by providing a one-time treatment that allows the liver to produce clotting factor IX, which is deficient in these patients. Once administered, this gene therapy aims to permanently correct the underlying genetic defect, enabling patients to maintain factor IX levels within a normal or near-normal range, thus addressing the root cause of the disorder.
Are there any risks associated with gene therapy for hemophilia?
As with any medical treatment, gene therapy for hemophilia comes with potential risks, including immune responses to the viral vector, liver inflammation, and other adverse effects. However, clinical trials for therapies like Hemgenix have shown promising safety profiles, and ongoing monitoring is crucial to manage any unexpected reactions post-treatment.
What is the expected recovery process after receiving gene therapy for hemophilia?
Recovery after receiving gene therapy for hemophilia, such as Hemgenix, generally involves close medical monitoring for side effects and assessing the body’s response to the treatment. Patients may receive follow-up care for liver function and factor IX levels, but many report significant improvements, often experiencing faster healing times and fewer bleeding events as their clotting factor levels normalize.
How does gene therapy impact the future of hemophilia treatment?
Gene therapy represents a groundbreaking advancement in hemophilia treatment, offering hope for a potential cure rather than ongoing symptom management. This innovative approach may shift the paradigm of care, allowing individuals with hemophilia to lead more normal lives without the constant burden of regular infusions of clotting factor therapy.
Is gene therapy for hemophilia covered by insurance?
Insurance coverage for gene therapy for hemophilia, such as Hemgenix, can vary significantly. While high treatment costs may pose a challenge, many insurance companies negotiate rates and may cover part or all of the therapy depending on individual circumstances, specific policies, and medical necessity. It’s essential for patients to consult with their healthcare provider and insurer to understand coverage options.
| Aspects | Details |
|---|---|
| Patient Background | Terence Blue, diagnosed with hemophilia at a young age, has managed his condition for over 27 years, previously requiring frequent injections of clotting factors. |
| Gene Therapy Introduction | Blue received Hemgenix, a gene therapy for hemophilia B, which was FDA approved in November 2022, targeting the liver to produce clotting factor IX. |
| Treatment Overview | The treatment involves a single-dose therapy that uses targeted viruses to insert corrected genes into liver cells. |
| Market Challenges | High costs and limited patient interest pose challenges for developing and maintaining gene therapies on the market. |
| Patient Experience | Post-treatment, Blue experienced a significant increase in factor IX levels and reported faster healing times. |
Summary
Gene therapy for hemophilia has the potential to transform the lives of patients like Terence Blue by reducing their dependency on frequent injections of clotting factors. With the pioneering treatment Hemgenix, recent advancements in gene therapy are offering new hope to those suffering from hemophilia, allowing for improved treatment outcomes and enhanced quality of life. As these therapies continue to evolve, they may pave the way for a future where living with hemophilia is significantly less burdensome.